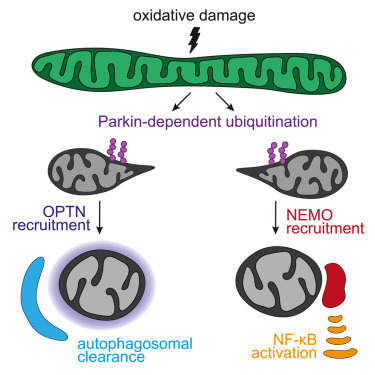
Dividing eukaryotic cells package extremely long chromosomal DNA molecules into discrete bodies to enable microtubule-mediated transport of one genome copy to each of the newly forming daughter cells1,2,3. Assembly of mitotic chromosomes involves DNA looping by condensin4,5,6,7,8 and chromatin compaction by global histone deacetylation9,10,11,12,13. Although condensin confers mechanical resistance to spindle pulling forces14,15,16, it is not known how histone deacetylation affects material properties and, as a consequence, segregation mechanics of mitotic chromosomes. Here we show how global histone deacetylation at the onset of mitosis induces a chromatin-intrinsic phase transition that endows chromosomes with the physical characteristics necessary for their precise movement during cell division. Deacetylation-mediated compaction of chromatin forms a structure dense in negative charge and allows mitotic chromosomes to resist perforation by microtubules as they are pushed to the metaphase plate. By contrast, hyperacetylated mitotic chromosomes lack a defined surface boundary, are frequently perforated by microtubules and are prone to missegregation. Our study highlights the different contributions of DNA loop formation and chromatin phase separation to genome segregation in dividing cells.
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur