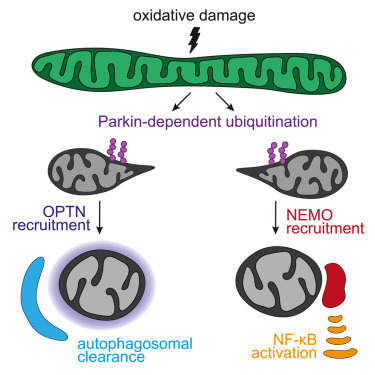
Impaired degradation of the transcriptional coactivator YAP1 and IL6ST (interleukin 6 cytokine family
signal transducer), two proteins deregulated in liver cancer, has been shown to promote tumor
growth. Here, we demonstrate that YAP1 and IL6ST are novel substrates of chaperone-mediated
autophagy (CMA) in human hepatocellular carcinoma (HCC) and hepatocyte cell lines. Knockdown of
the lysosomal CMA receptor LAMP2A increases protein levels of YAP1 and IL6ST, without changes in
mRNA expression. Additionally, both proteins show KFERQ-dependent binding to the CMA chaper–
one HSPA8 and accumulate into isolated lysosomes after stimulation of CMA by prolonged starva–
tion. We further show that LAMP2A downregulation promotes the proliferation and migration in HCC
cells and a human hepatocyte cell line, and that it does so in a YAP1- and IL6ST-dependent manner.
Finally, LAMP2A expression is downregulated, and YAP1 and IL6ST expression is upregulated, in
human HCC biopsies. Taken together, our work reveals a novel mechanism that controls the turnover
of two cancer-relevant proteins and suggests a tumor suppressor function of CMA in the liver,
advocating for the exploitation of CMA activity for diagnostic and therapeutic purpose.
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur