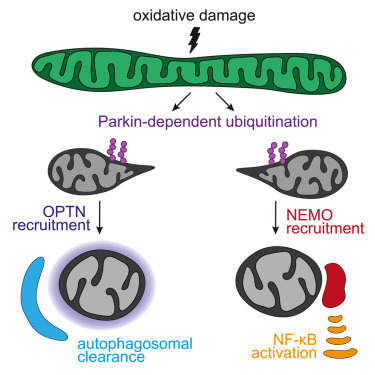
Serum- and glucocorticoid-regulated kinase 3 (Sgk3) is a serine/threonine protein kinase activated by the phospholipid phosphatidylinositol 3-phosphate (PI3P) downstream of growth factor signaling via class I phosphatidylinositol 3-kinase (PI3K) signaling and by class III PI3K/Vps34-mediated PI3P production on endosomes. Upregulation of Sgk3 activity has recently been linked to a number of human cancers; however, the precise mechanism of activation of Sgk3 is unknown. Here, we use a wide range of cell biological, biochemical, and biophysical techniques, including hydrogen–deuterium exchange mass spectrometry, to investigate the mechanism of activation of Sgk3 by PI3P. We show that Sgk3 is regulated by a combination of phosphorylation and allosteric activation. We demonstrate that binding of Sgk3 to PI3P via its regulatory phox homology (PX) domain induces large conformational changes in Sgk3 associated with its activation and that the PI3P-binding pocket of the PX domain of Sgk3 is sequestered in its inactive conformation. Finally, we reconstitute Sgk3 activation via Vps34-mediated PI3P synthesis on phosphatidylinositol liposomes in vitro. In addition to identifying the mechanism of Sgk3 activation by PI3P, our findings open up potential therapeutic avenues in allosteric inhibitor development to target Sgk3 in cancer.
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur
Mol Cell 2023 Sep 7;83(17):3188-3204.e7
O. Harding, E. Holzer, J.F. Riley, S. Martens, E.L.F. Holzbaur