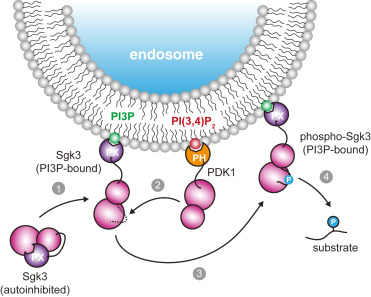
3-phosphoinositide-dependent kinase 1 (PDK1) is an essential serine/threonine protein kinase, which plays a crucial role in cell growth and proliferation. It is often referred to as a ‘master’ kinase due to its ability to activate at least 23 downstream protein kinases implicated in various signaling pathways. In this study, we have elucidated the mechanism of phosphoinositide-driven PDK1 auto-activation. We show that PDK1 trans-autophosphorylation is mediated by a PIP3-mediated face-to-face dimer. We report regulatory motifs in the kinase-PH interdomain linker that allosterically activate PDK1 autophosphorylation via a linker-swapped dimer mechanism. Finally, we show that PDK1 is autoinhibited by its PH domain and that positive cooperativity of PIP3 binding drives switch-like activation of PDK1. These results imply that the PDK1-mediated activation of effector kinases, including Akt, PKC, Sgk, S6K and RSK, many of whom are not directly regulated by phosphoinositides, is also likely to be dependent on PIP3 or PI(3,4)P2.
J Biol Chem 297(2) 100919
D. Pokorny, L. Truebestein, K. D. Fleming, J. E. Burke, and T. A. Leonard
J Biol Chem 297(2) 100919
D. Pokorny, L. Truebestein, K. D. Fleming, J. E. Burke, and T. A. Leonard