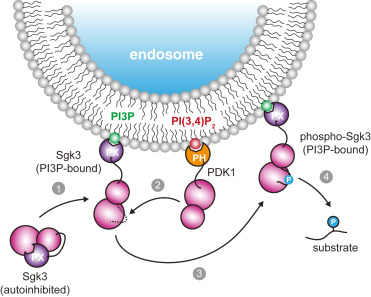
Many eukaryotic protein kinases are activated by phosphorylation of their activation loop. Some protein kinases can autoactivate by phosphorylating their own activation loop. This reaction is widely believed to occur exclusively in trans (by a copy of the same protein) and has been observed in a wide array of kinases that undergo stimulus-induced dimerization. Here, we describe the inverse mechanism for activation of protein kinase D (PKD), an essential kinase involved in membrane trafficking. We show that inactive PKD is dimeric and that its activation depends on dissociation of its kinase domains followed by activation loop autophosphorylation in cis (by itself). Nature has, therefore, found two solutions to the same problem that are simply the inverse of one another.
J Biol Chem 297(2) 100919
D. Pokorny, L. Truebestein, K. D. Fleming, J. E. Burke, and T. A. Leonard
J Biol Chem 297(2) 100919
D. Pokorny, L. Truebestein, K. D. Fleming, J. E. Burke, and T. A. Leonard